What to Expect at the 5th In Vivo Cell Engineering & Gene Editing Summit
The 5th In Vivo Cell Engineering & Gene Editing Summit brings together the researchers, translational leaders, and strategic decision-makers driving the next stage of in vivo innovation.
Designed for teams navigating real scientific and operational challenges, the meeting offers a focused environment to explore delivery engineering, early clinical signals, CMC scale‑up, and global development pathways with peers facing the same obstacles.
Over three days, attendees will have access to detailed case studies, collaborative problem‑solving sessions, and open discussions that delve into practical questions about targeting, off‑tissue activity, manufacturability, and regulatory readiness. With curated networking, working groups, and the new Partnerships Mixer, the summit makes it easy to connect with biotechs, pharma, and solution providers advancing programs worldwide.
Whether building foundational delivery work or preparing an IND, this is where in vivo developers sharpen their strategy and move forward with clarity.

The Summit Will Cover:
Targeting & Delivery Deep Dives
Gain practical insight into how developers are selecting, optimizing, and comparing delivery vehicles across LNPs, VLPs, LVVs, and emerging nonviral platforms, with real examples of biodistribution, payload performance, and extrahepatic targeting.
Translational Strategies Grounded in Real Data
Explore how teams are interpreting early human signals, biomarker readouts, immune kinetics, and safety observations to refine dose selection, adjust monitoring plans, and strengthen IND enabling evidence.
CMC, Scale-Up & Platform Guidance
Hear from experts tackling the operational side of in vivo development, including analytical readiness, comparability expectations, vector and LNP manufacturing, and the practical steps needed to move from early research toward clinical supply.
Global Development Intelligence
Understand how developers are shaping multinational strategies across the US, Europe, and Asia, including IIT design, regulatory touchpoints, site selection, and the operational considerations that influence speed and cost.
What's New for 2026?
Dedicated Spotlight on In Vivo Development in Asia
A full workshop focusing on IIT experiences, regional regulatory expectations, manufacturing capabilities and early clinical outcomes across China and emerging APAC hubs.
New Investor & M&A Insights
Fresh discussions on valuation drivers, differentiation, and the dealmaking trends shaping the in vivo competitive landscape.
Enhanced Networking Format
The introduction of the Partnerships Mixer and more facilitated discussion sessions to help attendees build commercially meaningful relationships.
Industry Momentum is Building
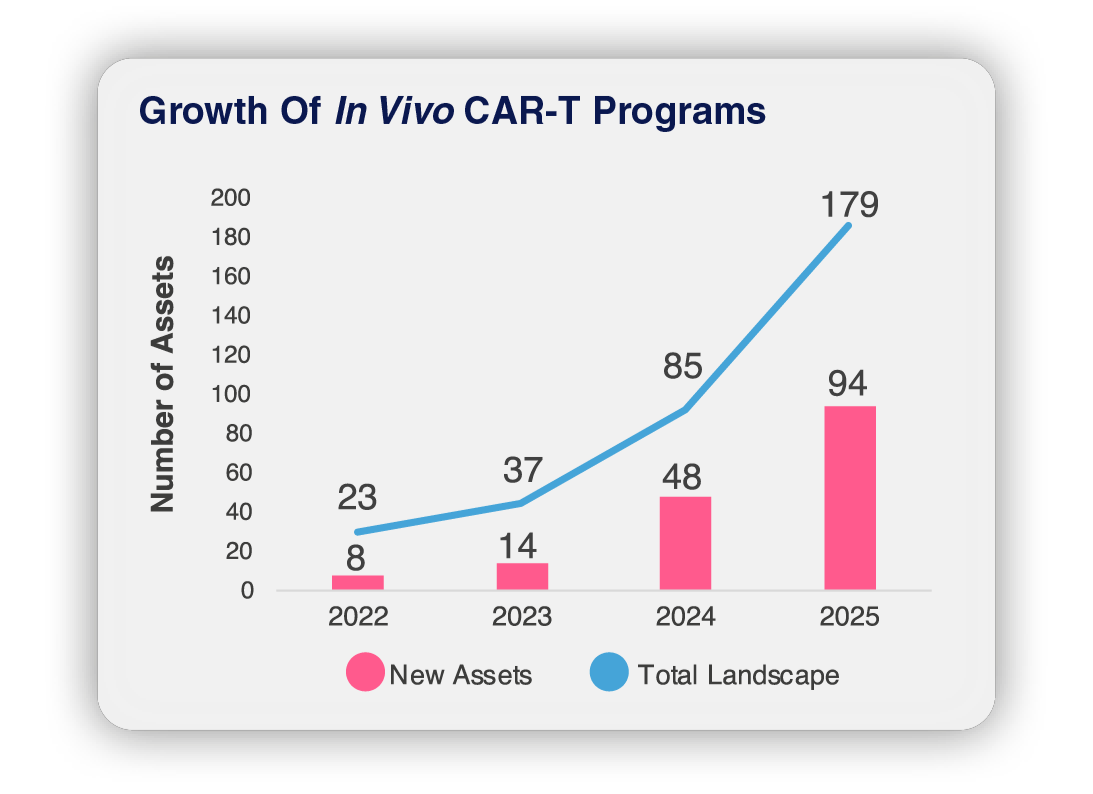
Pharma’s surge into in vivo cell engineering and gene editing products has pushed the field to a potential inflection point. With over $7.3B in in vivo acquisitions in the past year, alongside first-in-human signals and rapid modality diversification, momentum is accelerating. Companies are expanding pipelines, speeding up clinical timelines, and competing to develop in vivo platforms that can scale globally.
The field is now expanding beyond traditional indications with more assets targeting solid tumors and autoimmune diseases, enabling access to wider patient and commercial opportunities.
Attending Companies Include







